We design materials with theories, computations and data.
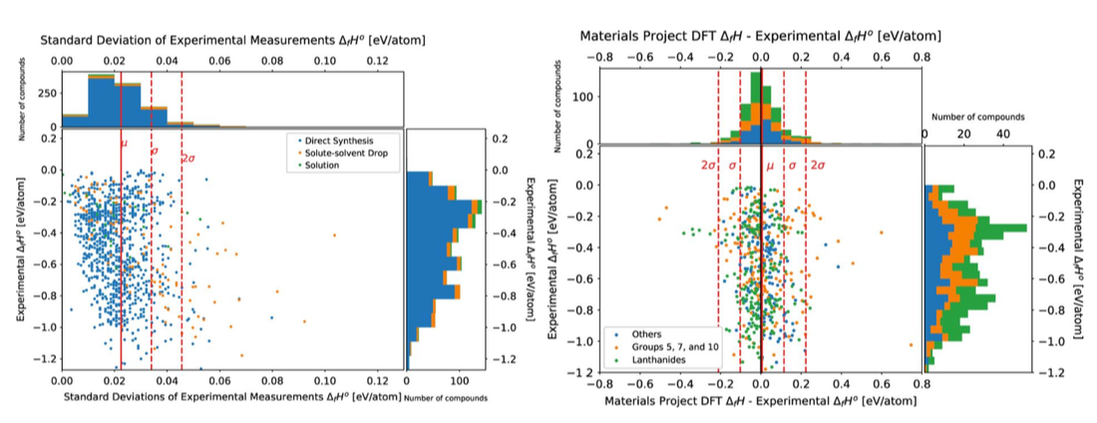
An open database of experimental formation enthalpy data for > 1200 metallic phases released10/18/2017 The standard enthalpy of formation is a fundamental thermodynamic property that determines the phase stability of a compound, which can be coupled with other thermodynamic data to calculate phase diagrams. Calorimetry provides the only direct method by which the standard enthalpy of formation is experimentally measured. However, the measurement is often a time and energy intensive process. We present a dataset of enthalpies of formation measured by high-temperature calorimetry. The phases measured in this dataset include intermetallic compounds with transition metal and rare-earth elements, metal borides, metal carbides, and metallic silicides. The dataset contains 1,276 entries on experimental enthalpy of formation values and structural information. Most of the entries are for binary compounds but ternary and quaternary compounds are being added as they become available.
The dataset also contains predictions of enthalpy of formation from first-principles calculations for comparison. We compared DFT formation enthalpy values from the Materials Project and OQMD, and identified problematic systems that show substantial discrepancies between experiments and PBE-DFT. The most recent database can be queried from our website: http://tptc.iit.edu/ The data file can be accessed from Figshare: https://doi.org/10.6084/m9.figshare.c.3822835 Experimental formation enthalpies for intermetallic phases and other inorganic compounds", Scientific Data, 4, 170162 (2017) [PDF]
0 Comments
|
Categories
All
Archives
September 2020
|

 RSS Feed
RSS Feed
