|
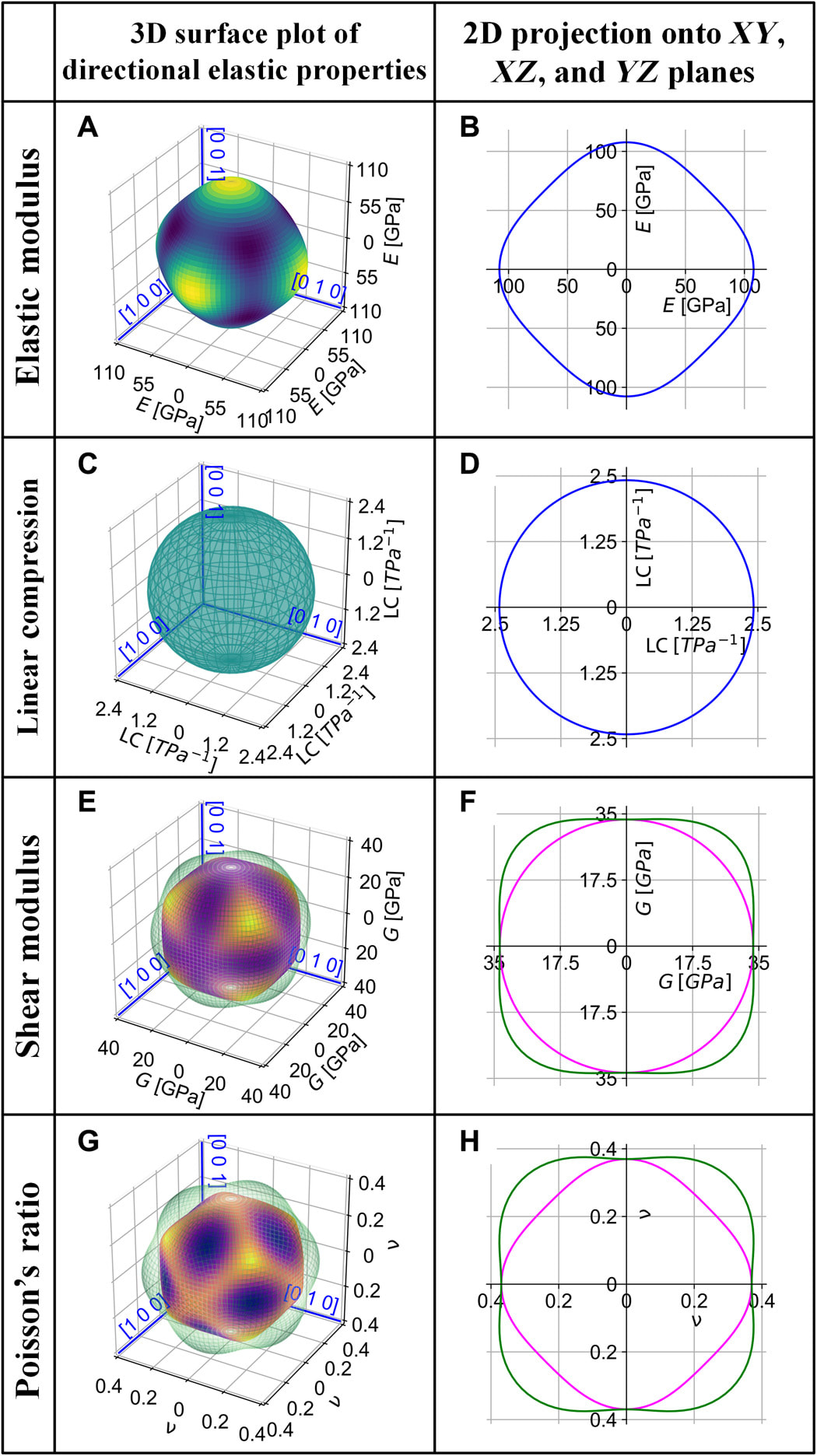
We recently discovered some surprising properties in the NbTaTiV high-entropy alloys. For example, the alloy shows good elastic isotropy at room temperature, but with the increase of temperature, its elastic anisotropy actually increases. Such temperature dependence of elastic anisotropy is not induced by phase transformations, but is an intrinsic feature of the BCC high entropy alloy. Our first-principles and machine learning predictions are in good agreement with experimental observations. These results are reported in an article on Science Advances.
The study was in collaboration with Prof. Peter Liaw's group at University of Tennessee and Prof. Yi-Chia Chou's group at National Chiao Tung University in Taiwan.
0 Comments
Excited to receive a NSF CAREER award on first-principles and machine learning studies of high-entropy alloys! The project will be jointly supported by the Division of Materials Research and the NSF Office of Advanced Cyberinfrastructure.
https://www.nsf.gov/awardsearch/showAward?AWD_ID=1945380 We are excited to be part of a team awarded a 2 million NSF grant on developing interpretable augmented intelligence for multiscale materials discovery. The multi-disciplinary project will be in collaboration with research groups at University of Colorado Boulder (Hendrik Heinz), Ohio State University (Yusu Wang), Johns Hopkins University (Yanxun Xu), and Columbia University (Steve Sun).
https://www.nsf.gov/awardsearch/showAward?AWD_ID=1940114
We are glad to receive another grant from Wanger Institute for Sustainable Energy Research (WISER) to develop bi-functional heteroatom catalysts for high efficiency and long cycle life lithium-air batteries. The work will be in collaboration with Prof. Mohammad Asadi and Prof. Reza Shahbazian-Yassar (UIC).
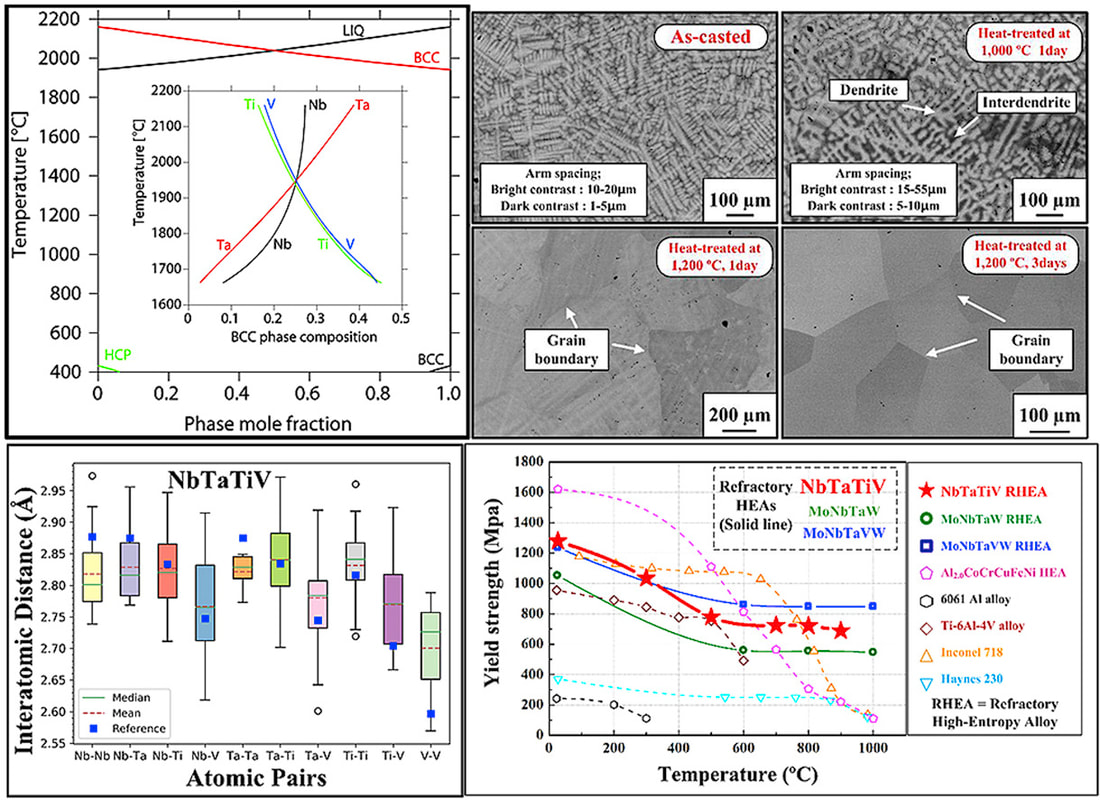
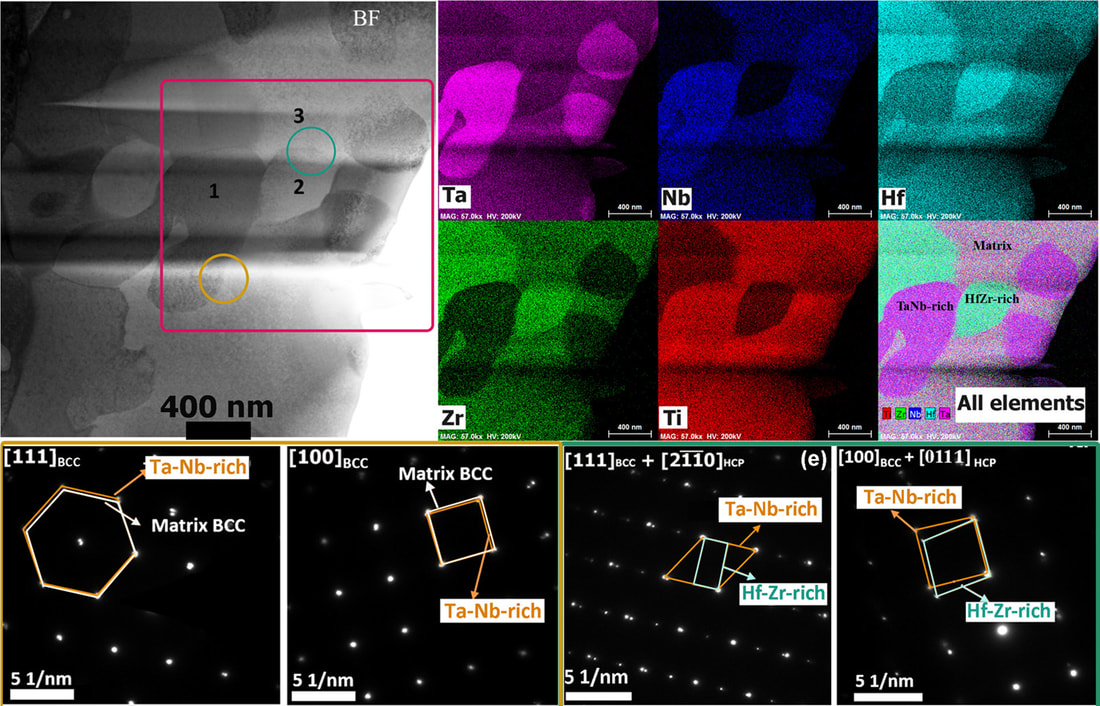
In collaboration with Prof. Peter Liaw's group at University of Tennessee, we recently designed a new single-phase BCC refractory high-entropy alloy (HEA) NbTaTiV. The novel HEA exhibits high yield strength and ductility at both room and high temperatures. First-principles approaches based on density functional theory (DFT) provided critical information on the thermodynamic stability and lattice distortions in the alloy design. Integrated with the CALPHAD method and in situ structural characterizations, a heat-treatment process was developed that eliminates structural and chemical inhomogeneity. "Lattice distortion in a strong and ductile refractory high-entropy alloy", Acta Materialia, 160, 158-172 (2018) [PDF] Refractory high-entropy alloys (RHEA) are promising structural materials for high-temperature thermal-harsh environment. The HfNbTaTiZr RHEA is a good example that shows a rare combination of high strength and good ductility. In real-world applications, high-temperature alloys have to maintain a high phase stability not only at high temperatures, but also for a wide range of temperatures for a prolonged service time. While the HfNbTaTiZr RHEA is generally regarded as a single-phase BCC solid solution, recent studies by Senkov et al suggest phase decomposition after cold-rolling at 800 °C. Controversy still exists for the phase stability of the HfNbTaTiZr RHEA at intermediate temperatures. In the present work, we investigated the phase decomposition of the RHEA at different temperatures (500–1000 °C). The formation of BCC Ta-Nb-rich and HCP HfZr-rich precipitates, as well as their preferred orientation to the BCC matrix, are elucidated from experiments. Thermodynamic modeling shows good agreement with experiments.
"Phase transformations of HfNbTaTiZr high-entropy alloy at intermediate temperatures", Scripta Materialia, 158, 50-56 (2019) [PDF] We are glad to receive a grant from Wanger Institute for Sustainable Energy Research (WISER) to develop advanced catalysts for photo-electrocatalytic conversion of CO2 to energy rich fuels. The work will be in collaboration with Prof. Mohammad Asadi, Prof. Carlo Segre, and Prof. Reza Shahbazian-Yassar (UIC).
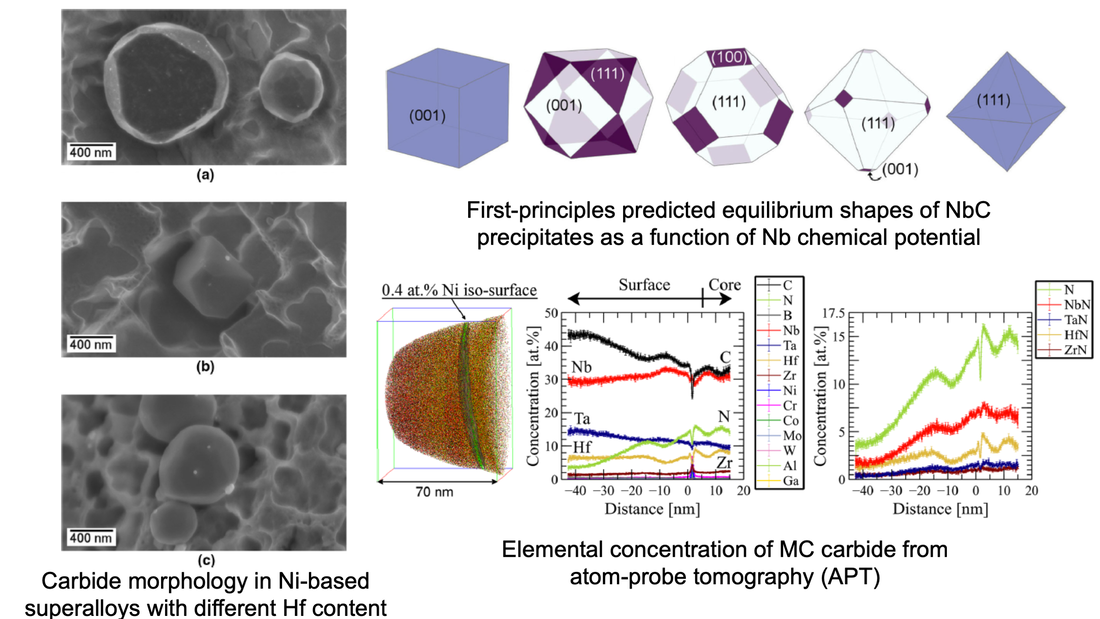
Carbide precipitates in Ni-based superalloys are often desirable phases that can improve high-temperature properties as well as aid in microstructural refinement of the material; however, they can also serve as crack initiation sites during fatigue. To date, the knowledge on carbide formation has mostly originated from assessments of cast and wrought Ni-based superalloys. As powder-processed Ni-based superalloys are becoming increasingly widespread, understanding the different mechanisms by which they form becomes increasingly important. In the present work, we performed detailed characterization of MC carbides present in two experimental high Nb-content powder-processed Ni-based superalloys and revealed that Hf additions affect the resultant carbide morphologies. This morphology difference was attributed to a higher magnitude of elastic strain energy along the interface associated with Hf being soluble in the MC carbide lattice. The characterization results of the segregation behavior of Hf in the MC carbides and the subsequent influence on their morphology were compared to density functional theory calculations and found to be in good agreement, suggesting that computational modeling can successfully be used to predict carbide features.
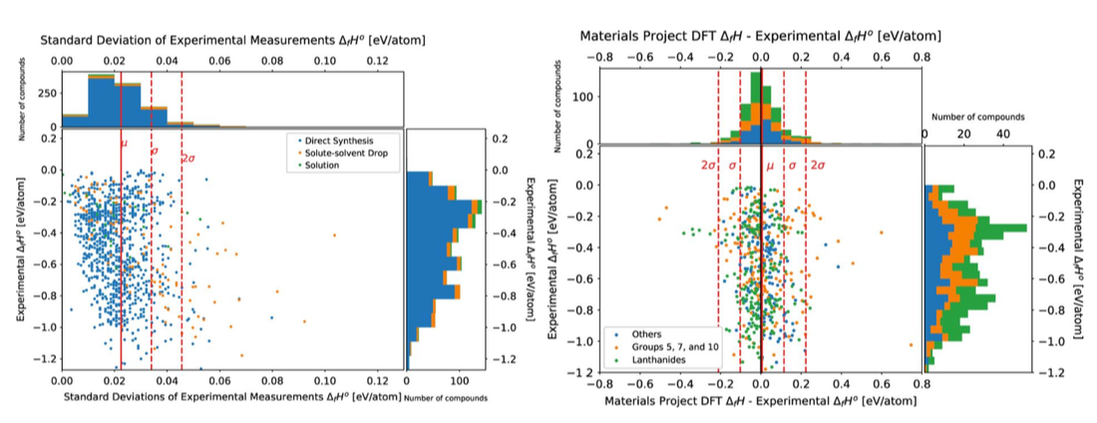
"MC carbide characterization in high refractory content powder-processed Ni-based superalloys", Metallurgical and Materials Transactions A, 49, 2340-2351 (2018) [PDF] An open database of experimental formation enthalpy data for > 1200 metallic phases released10/18/2017 The standard enthalpy of formation is a fundamental thermodynamic property that determines the phase stability of a compound, which can be coupled with other thermodynamic data to calculate phase diagrams. Calorimetry provides the only direct method by which the standard enthalpy of formation is experimentally measured. However, the measurement is often a time and energy intensive process. We present a dataset of enthalpies of formation measured by high-temperature calorimetry. The phases measured in this dataset include intermetallic compounds with transition metal and rare-earth elements, metal borides, metal carbides, and metallic silicides. The dataset contains 1,276 entries on experimental enthalpy of formation values and structural information. Most of the entries are for binary compounds but ternary and quaternary compounds are being added as they become available.
The dataset also contains predictions of enthalpy of formation from first-principles calculations for comparison. We compared DFT formation enthalpy values from the Materials Project and OQMD, and identified problematic systems that show substantial discrepancies between experiments and PBE-DFT. The most recent database can be queried from our website: http://tptc.iit.edu/ The data file can be accessed from Figshare: https://doi.org/10.6084/m9.figshare.c.3822835 Experimental formation enthalpies for intermetallic phases and other inorganic compounds", Scientific Data, 4, 170162 (2017) [PDF] |
Categories
All
Archives
September 2020
|


 RSS Feed
RSS Feed
